Question; Need to double-check... (1 Viewer)
- Thread starter dsakvyilsa
- Start date
dsakvyilsa
Well-Known Member
Come to think of it, a few of the answers don't really seem right..... would someone please have a look through and tell me what they think should be the right answers? Thanks
Attachments
-
184.3 KB Views: 9
The good thing is, all of these questions happen to be questions from past HSC exams.
The question in your first attachment is Question 16 from the 2017 Chemistry HSC exam. The following is an explanation to the answer:
The third question in the PDF document is Question 31 (b) from the 2017 Chemistry HSC exam. Part (i) can be done as follows:
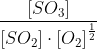
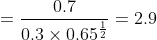
Since 2.9 ≠ 12.1, equilibrium has not been reached.
The fourth question in the PDF document is Question 18 from the 2017 Chemistry HSC exam. The following is an explanation to the answer:
_{3\left(aq\right)}+Na_2SO_{4\left(aq\right)}\rightarrow BaSO_{4\left(s\right)}+2NaNO_{3\left(aq\right)})
=c\times V=0.2\times 0.2=0.04\:\text{mol})
=0.04\:\text{mol}\:\left(1:1\:\text{mole ratio}\right))
=n\times MM=0.04\times \left(137,3+32.07+16\times 4\right)=9.3348\:g\:=9.33\:g\:\left(3\:\text{s. f.}\right))
The answer is therefore (C).
The sixth question in the PDF document is Question 14 from the 2016 Chemistry HSC exam. Essentially, decreasing the volume causes the equilibrium to shift to the side with less gas moles i.e. towards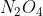 to minimise the disturbance (Le Chatelier’s principle). The answer is therefore (B).
to minimise the disturbance (Le Chatelier’s principle). The answer is therefore (B).
I hope this helps!
The question in your first attachment is Question 16 from the 2017 Chemistry HSC exam. The following is an explanation to the answer:
The first question in the PDF document that you attached is Question 8 from the 2017 Chemistry HSC exam. The answer is (C) because barium does not precipitate with chloride but lead does.Explanation said:(not directly in the equation but NaOH causes the depletion of the H2co3 through neutralisation and thus causes the equilibrium to shift right reducing co2 concentration and thus the gas pressure is reduced ) (can't be A as adding more CO2 does shift equilibrium right reducing gas moles i.e. pressure but remember that Le Chatelier's principle only minimises the change and doesn't complete counteract it. So in fact you are increasing pressure by adding more carbon dioxide. Can't be c as this will shift equilibrium left and increase the pressure. Can't be D as increasing temperature shifts equilibrium to the endothermic side (left) increases co2 gas and thus pressure)
The answer is therefore (B).
The third question in the PDF document is Question 31 (b) from the 2017 Chemistry HSC exam. Part (i) can be done as follows:
Part (b) can be answered as follows:Explanation said:
| Moles | Moles | Moles | |
| Initial | 1.0 | 1.0 | 0 |
| Used/made | 0.70 | 0.35 | 0.70 |
| Final | 0.30 | 0.65 | 0.70 |
Since 2.9 ≠ 12.1, equilibrium has not been reached.
The fourth question in the PDF document is Question 18 from the 2017 Chemistry HSC exam. The following is an explanation to the answer:
The fifth question in the PDF document is Question 19 from the 2016 Chemistry HSC exam. It can be done as follows:Explanation said:(Y and Z on the same side of the equation and X is on there side since both Y and Z increase while X decreases and this is because the change that occurs is due to a change in pressre/volume (increase in volume) as all concentrations drop instantaneously together. By LCP the system will shift to the side with the most gas moles to compensate for the reduced pressure. So this side must be the Y and Z one as these go up in concentration. So summarising Y and Z on the same side, and the side with Y and Z must have more gas moles. Now consider the ratio concentration changes for molar ratios (i.e. they all go up/down by the same one unit displayed in answer D)
The answer is therefore (D).
The answer is therefore (C).
The sixth question in the PDF document is Question 14 from the 2016 Chemistry HSC exam. Essentially, decreasing the volume causes the equilibrium to shift to the side with less gas moles i.e. towards
I hope this helps!
dsakvyilsa
Well-Known Member
thankyou for this!!!! a couple of the answers we were given were incorrect from what I can see so far, I think some questions were removed/changed and the answer sheet didn't really align (it had answers for questions we werent given and some of the multiple choice answers were back to front)The good thing is, all of these questions happen to be questions from past HSC exams.
The question in your first attachment is Question 16 from the 2017 Chemistry HSC exam. The following is an explanation to the answer:
The first question in the PDF document that you attached is Question 8 from the 2017 Chemistry HSC exam. The answer is (C) because barium does not precipitate with chloride but lead does.
The third question in the PDF document is Question 31 (b) from the 2017 Chemistry HSC exam. Part (i) can be done as follows:
Part (b) can be answered as follows:
Moles Moles Moles Initial 1.0 1.0 0 Used/made 0.70 0.35 0.70 Final 0.30 0.65 0.70
Since 2.9 ≠ 12.1, equilibrium has not been reached.
The fourth question in the PDF document is Question 18 from the 2017 Chemistry HSC exam. The following is an explanation to the answer:
The fifth question in the PDF document is Question 19 from the 2016 Chemistry HSC exam. It can be done as follows:
The answer is therefore (C).
The sixth question in the PDF document is Question 14 from the 2016 Chemistry HSC exam. Essentially, decreasing the volume causes the equilibrium to shift to the side with less gas moles i.e. towardsto minimise the disturbance (Le Chatelier’s principle). The answer is therefore (B).
I hope this helps!
Last edited:
wizzkids
Well-Known Member
- Joined
- Jul 13, 2016
- Messages
- 339
- Gender
- Undisclosed
- HSC
- 1998
The solubility of CO2 (g) in water is highly pH dependent. At low pH, the solubility of CO2 (g) is low. At high pH, the solubility of CO2 (g) is high. In fact, sodium hydroxide solution can be used to sequester CO2(g) from the atmosphere.Hey guys, just wanted a second opinion on this one, the sample answer didn't seem right and I don't USUALLY have an issue with this type of question :'(
Thanks, sorry for the poor quality.
View attachment 37157
Last edited:


