-
Best of luck to the class of 2024 for their HSC exams. You got this! Let us know your thoughts on the HSC exams here -
YOU can help the next generation of students in the community! Share your trial papers and notes on our Notes & Resources page
water ionisation (1 Viewer)
- Thread starter sssona09
- Start date
captainhelium
water enthusiast
The Bronsted-Lowry definition of acids and bases states that acids are considered as proton donors and bases are considered as proton acceptors.can someone please explain tis equation in terms of water ionisation?
H2O (l) -->H+ (aq) + OH-(aq)
also, if acid ionises in water, then it gives up a proton - so how do we get H3O+? shouldn't it be H3o-
For example, consider the acid ionisation equation for hydrochloric acid in water:
HCl(aq) + H2O --> H3O+ +Cl-
In the above reaction, HCl donates a proton (H+) to water. Remember that when HCl is an aqueous solution, it dissociates into its respective ions, that is
HCl --> H+ + Cl-.
Essentially, this H+ ion from the dissociation of HCl will be given to the water molecule. Thus, water gains an extra hydrogen ion, which is positively charged (since hydrogen ions are essentially protons), which is why water is considered a base in the acid ionisation equation since it accepts a proton.
So basically, with H2O accepting a proton (H+) from HCl, you get hydronium which is H3O+. Notice how hydronium has an extra hydrogen atom compared to water and is positively charged? This is due to water accepting the H+ from HCl.
Now since HCl has donated a proton, all it has left is a chloride ion (from the equation regarding the dissociation of HCl). Thus, the products of an acid ionisation equation are hydronium and some random anion.
Thus we get the equation,
HCl + H2O --> H3O+ + Cl-
Hopefully that made more sense about why H3O+ is formed.
Also, regarding your question about explaining water ionisation, essentially water ionises with itself in water, which sounds kinda funny when you first hear about it. Consider it like an interaction between an acid and a base -
H2O + H2O --> H3O+ + OH-
So in the above equation, one of the water molecules will act as an acid and the other will act as a base. For example, let's say that the first water molecule is an acid.
This means that it will 'lose' one of its protons (H+ ion) and give it to the other water molecule. Thus, when one of the H2O molecule loses and donates a positive H+, it becomes OH- (since OH- has one less H+ than water).
This donated proton gets accepted by the other H2O molecule and so becomes H3O+. Thus we get the equation,
H2O + H2O --> H3O+ + OH-.
Essentially, one of the water molecules acts as an acid (since it donates a proton) and the other water molecule acts as a base (since it is accepting a proton).
The equation H2O --> H+ + OH- is really just a simplification of the above equation without considering the Bronsted-Lowry definitions I think.
Also, just remember that the self-ionisation of water is a reversible reaction
Hope that helped! Haven't touched Chemistry since my HSC exam so I might be a bit fuzzy on the info.
Last edited:
dan964
what
Can agree, captain helium is on point with regards to the theory.
Water ionization would be an equilibrium reaction

Remember that when we talk about donating a proton, it is equivalent to talking about donating a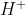 ion.
ion.
The equation would be


Combining these two equations gives the full ionization equation for water which is reversible.
Water ionization would be an equilibrium reaction
Remember that when we talk about donating a proton, it is equivalent to talking about donating a
The equation would be
Combining these two equations gives the full ionization equation for water which is reversible.
Last edited:
Protons have a positive charge so that's why the hydronium ion (h3o+) is positivecan someone please explain tis equation in terms of water ionisation?
H2O (l) -->H+ (aq) + OH-(aq)
also, if acid ionises in water, then it gives up a proton - so how do we get H3O+? shouldn't it be H3o-
Sent from my Pixel using Tapatalk

