Make a Difference – Donate to Bored of Studies!
Students helping students, join us in improving Bored of Studies by donating and supporting future students!
how are ions atomised in a flame test??? (1 Viewer)
- Thread starter dumNerd
- Start date
@jazz519View attachment 29829
This literally states that the metal ion is turned into a metal atom when subjected to a flame, how is this possibe?
Everwinter
Member
- Joined
- Oct 5, 2020
- Messages
- 49
- Gender
- Male
- HSC
- 2021
Wait, I thought atoms emit colours because the electrons become excited. I have no idea how did ions gett involved.
basically when an orginally aqueous solution is heated, the water evaporates which would normally leave behind ions, however this is saying that these ions are atomised, meaning they somehow gain electrons and become balanced atoms. What you say is correct but that is not the question I'm asking (what you say is a bit flawed but I assume you know it)Wait, I thought atoms emit colours because the electrons become excited. I have no idea how did ions gett involved.
qUiCkMaThS
New Member
- Joined
- Aug 6, 2020
- Messages
- 6
- Gender
- Undisclosed
- HSC
- 2019
I think what they are trying to say is that the valence electron from the metal cation is excited i.e. 1 of the e- goes from the 2p6 state into 3s1, which is similar to the energy level configuration of the respective metal atom, but that's wrong to say because an ion is formed by the lack/ abundance of e- and is not determined by the number of energy levels. IMO I wouldn't use this wording in an answer because its flawed and confusing. What source are you quoting from? I would suggest using a textbook or Khan academy, because they explain concepts in a much better way.
Also, aren't you starting year 11 this year? Don't worry about studying now, just relax
Also, aren't you starting year 11 this year? Don't worry about studying now, just relax
This is quoted from ACE chemistry tutoringI think what they are trying to say is that the valence electron from the metal cation is excited i.e. 1 of the e- goes from the 2p6 state into 3s1, which is similar to the energy level configuration of the respective metal atom, but that's wrong to say because an ion is formed by the lack/ abundance of e- and is not determined by the number of energy levels. IMO I wouldn't use this wording in an answer because its flawed and confusing. What source are you quoting from? I would suggest using a textbook or Khan academy, because they explain concepts in a much better way.
Also, aren't you starting year 11 this year? Don't worry about studying now, just relax
Everwinter
Member
- Joined
- Oct 5, 2020
- Messages
- 49
- Gender
- Male
- HSC
- 2021
The ions turn back into the ionic compound (salt or lattice of atoms) when the water is evaporated, they are no longer ions. Is this what you are confused at? (I also think the source is quite confusing)basically when an orginally aqueous solution is heated, the water evaporates which would normally leave behind ions, however this is saying that these ions are atomised, meaning they somehow gain electrons and become balanced atoms. What you say is correct but that is not the question I'm asking (what you say is a bit flawed but I assume you know it)
They cant just turn back into the ionic compound, where do they get the electrons from???The ions turn back into the ionic compound (salt or lattice of atoms) when the water is evaporated, they are no longer ions. Is this what you are confused at? (I also think the source is quite confusing)
username_2
Active Member
- Joined
- Aug 1, 2020
- Messages
- 116
- Gender
- Male
- HSC
- 2020
Hmmm... I think this explains it a bit better. Think of the atomiser as a spray bottle with a salt solution where the metal cation is dissociated and it is an individual atom encased within the "atomised solution" - like spray droplets with ion atoms. What the atomiser does is spread it over the flame so that more ions become exposed to the added energy allowing for more of the "flame colour" or the transition to becoming visible - like shining light through the normal spray from the bottle to produce a rainbow, except it is not diffraction but electron transitions (I might have confused u there but eh). Hope this helps. (NOTE: As far as I can tell, the atomiser doesn't heat the solution or evaporate the water, the flame does)
O: I see but the wording is so shit, makes it seem as though the ions are turning into atomsHmmm... I think this explains it a bit better. Think of the atomiser as a spray bottle with a salt solution where the metal cation is dissociated and it is an individual atom encased within the "atomised solution" - like spray droplets with ion atoms. What the atomiser does is spread it over the flame so that more ions become exposed to the added energy allowing for more of the "flame colour" or the transition to becoming visible - like shining light through the normal spray from the bottle to produce a rainbow, except it is not diffraction but electron transitions (I might have confused u there but eh). Hope this helps. (NOTE: As far as I can tell, the atomiser doesn't heat the solution or evaporate the water, the flame does)
Eagle Mum
Well-Known Member
- Joined
- Nov 9, 2020
- Messages
- 615
- Gender
- Female
- HSC
- N/A
When the solvent evaporates, you don’t have ions because there’s no medium in which electrons can move around. Initially, you have a solid crystal. With more heat energy after evaporation has occurred, the crystal structure breaks down and you have individual atoms (this process is atomisation).basically when an orginally aqueous solution is heated, the water evaporates which would normally leave behind ions, however this is saying that these ions are atomised, meaning they somehow gain electrons and become balanced atoms. What you say is correct but that is not the question I'm asking (what you say is a bit flawed but I assume you know it)
Ooo this makes the most sense tytyWhen the solvent evaporates, you don’t have ions because there’s no medium in which electrons can move around. Initially, you have a solid crystal. With more heat energy after evaporation has occurred, the crystal structure breaks down and you have individual atoms (this process is atomisation).
Everwinter
Member
- Joined
- Oct 5, 2020
- Messages
- 49
- Gender
- Male
- HSC
- 2021
I think you already understood from what username_2 and Eagle Mum said, just to make it more clear, so in ionic compound (salt), the metal atoms lose their electrons and become cations, while the non-metals gain these electrons and become anions. The atoms in ionic compounds are held together by the electrostatic attraction force between the positive cations and the negative anions. When the ionic compound is placed in water (assumedly dissoveable), the water molecules dissociates the cations and anions the from solid salt, and the cations and anions become free-floating ions in the aqueous solution.
In the flame test, the aqueous solution is sprayed onto the flame, the water in the droplets of the solution quickly evaporate by the flame, without the water molecules that dissociate the cations and the anions, they are converted back into ionic compounds (solids) by the electrostatic force. Then the metal atom in the ionic compound receives a specific amount of energy from the flame and the electrons become excited and the atoms later emit light when the atoms return to their ground state (I think you already understand the detailed process from here)
*note: this would not be thorough enough in an actual response, but I think it is enough for you to understand the situation here.
In the flame test, the aqueous solution is sprayed onto the flame, the water in the droplets of the solution quickly evaporate by the flame, without the water molecules that dissociate the cations and the anions, they are converted back into ionic compounds (solids) by the electrostatic force. Then the metal atom in the ionic compound receives a specific amount of energy from the flame and the electrons become excited and the atoms later emit light when the atoms return to their ground state (I think you already understand the detailed process from here)
*note: this would not be thorough enough in an actual response, but I think it is enough for you to understand the situation here.
Last edited:
just one more thing to note.Ooo this makes the most sense tyty
the ion structure is not an ionic solid, so the bunsen burner can actually separate the ions from each other by destroying water. i doubt an ionic solid would be separated by a bunsen burner.
Eagle Mum
Well-Known Member
- Joined
- Nov 9, 2020
- Messages
- 615
- Gender
- Female
- HSC
- N/A
The maximum temperature of a Bunsen burner (at the tip of the blue part of the flame) can reach 1500 deg C. The thermal stability (ie. decomposition temperatures) of many common metal salts are below this temperature.just one more thing to note.
the ion structure is not an ionic solid, so the bunsen burner can actually separate the ions from each other by destroying water. i doubt an ionic solid would be separated by a bunsen burner.
The notes being quoted here are not well-written. The species giving rise to the colours in a flame test are indeed cations of the metal. There is confusion arising because an ionised atom is still an atom, just one that carries a net charge. Doing a flame test on sodium chloride does not involve any uncharged Na atoms with a 3s1 electron in its ground state, but rather Na+ cations.
Eagle Mum
Well-Known Member
- Joined
- Nov 9, 2020
- Messages
- 615
- Gender
- Female
- HSC
- N/A
The characteristic yellow colour from sodium in a flame test is from the excitation of its outermost electron and subsequent decay from the 3p orbit to 3s orbit. The sodium cation by definition does not possess this outer electron, so whilst in practice, the sample in the flame is quite likely a mixture of ions and atoms due to incomplete atomisation, my understanding is that it is only the atom, not the ion, which contributes to the visible yellow colour.The notes being quoted here are not well-written. The species giving rise to the colours in a flame test are indeed cations of the metal. There is confusion arising because an ionised atom is still an atom, just one that carries a net charge. Doing a flame test on sodium chloride does not involve any uncharged Na atoms with a 3s1 electron in its ground state, but rather Na+ cations.
Other electrons within both species (atoms & ions) will also be excited up to and subsequently decay from higher orbits, but due to their closer proximity to the positive nucleus, the much greater energy required for these other electrons to jump up to a higher orbit produces electromagnetic radiation, during orbital decay, of higher frequencies so they are outside the visible range.
Last edited:
@Eagle Mum, you are correct that the sodium D lines correspond to a transition between the 3s and 3p orbitals and it is this transition that is responsible for the flame test colour and the yellow sodium vapour lamps.
However, the fact that sodium atoms in a vapour lamp have an excitation from the 3s to the 3p orbital and the subsequent reverse transition leads to the emission does not mean that the excitation must have been an electron from the 3s orbital of a ground-state uncharged sodium atom to its 3p orbital.
1. Both an uncharged sodium atom and a sodium cation will give a yellow colour in a flame test
The selection rules for symmetry-allowed and symmetry-forbidden transitions in electronic spectroscopy are determined by the transition moment integral:
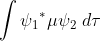
Specifically, if the transition moment function, is antisymmetric (that is, the function is odd) then the integral is zero and the transition is forbidden. If the wavefunctions relate to a species that has an inversion centre then the transition moment operator
is antisymmetric (that is, the function is odd) then the integral is zero and the transition is forbidden. If the wavefunctions relate to a species that has an inversion centre then the transition moment operator 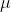 for an electric dipole transition has u symmetry and so is odd (recall: u is an abbreviation for ungerade, the German for "odd"). Then, any transition in which
for an electric dipole transition has u symmetry and so is odd (recall: u is an abbreviation for ungerade, the German for "odd"). Then, any transition in which  and
and 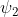 have the same symmetry will yield a triple product that is odd and a transition that is symmetry-forbidden. Both a single uncharged atom of sodium (or any other element) and a monatomic ion are spherical species and thus have an inversion centre. Consequently, in these species, transitions between orbitals of the same type of sub-shell are symmetry-forbidden as the excited and ground state wavefunctions will have the same symmetry, whether that be u symmetry of a p orbital or the g symmetry (g = gerade, "even") of an s or d orbital.
have the same symmetry will yield a triple product that is odd and a transition that is symmetry-forbidden. Both a single uncharged atom of sodium (or any other element) and a monatomic ion are spherical species and thus have an inversion centre. Consequently, in these species, transitions between orbitals of the same type of sub-shell are symmetry-forbidden as the excited and ground state wavefunctions will have the same symmetry, whether that be u symmetry of a p orbital or the g symmetry (g = gerade, "even") of an s or d orbital.
In other words, quantum mechanics dictates that transitions s to s, p to p, d to d, and f to f, are all forbidden on symmetry grounds in a spherical species. An excitation of an electron in a 2p orbital in a sodium cation is thus most likely to be 2p to 3s or 2p to 3d. An excitation from an already-excited sodium cation with a 3s electron is most likely to be 3s to 3p. The only possible relaxation pathway for an excited state sodium cation with a 3p electron is 3p to 3s unless there is (somehow) a vacancy in a 2s or 1s orbital. An excited state sodium cation with a 3d electron can relax 3d to 3p or 3d to 2p but not 3d to 3s.
In reality, spin-forbidden transitions are not necessarily impossible because vibrational coupling can alter the symmetry sufficiently for them to become weakly allowed, but that is not important in this case. Also, since these selection rules apply equally to a sodium atom as they do to a sodium cation, nothing in the above prevents uncharged sodium atoms present in a flame from producing a yellow colour - in fact, if uncharged sodium atoms are present, they will produce a colour. What the rules do explain is why sodium cations will also produce a yellow colour if present in a flame - though there is no 3s electron in the ground state, that does not mean that excitations can't be 2p to 3s to 3p and reverse, to give the flame colour, with an alternative being a 2p to 3d excitation with a 3d to 3p to 3s to 2p relaxation pathway.
2. Could we have uncharged sodium atoms in a flame?
If there is sodium vapour around, as in a sodium vapour lamp, sure. If there is sodium metal around, also sure - metallic sodium melts at 98 degC and boils 883 degC, so a bunsen is easily hot enough to turn Na(s) into Na(g). However, sodium metal oxidises rapidly in air and sodium oxide (Na2O) melts at 1132 degC and sublimes around 1275 degC. Even if sodium metal is present, placing it into a flame with an ample supply of air guarantees it will become sodium ions as part of an oxide rapidly.
For a flame test with sodium chloride, the question that @dumNerd asks is, for me, critical... if sodium chloride were somehow producing uncharged sodium atoms in a flame test, where is the electron coming from to reduce the sodium cation?
2 Na+ + 2 Cl- -----> 2 Na + Cl2 might seem plausible at first, but:
In short, the sodium cation is much more stable than sodium metal because the latter is easily oxidised to the former, even in air at room temperature. In a high-energy environment like a flame, short-lived and unstable species can be formed but it is difficult to see how or why sodium cations would be reduced unless something even more readily oxidised were present - and any such would surely be oxidised by air long before a sodium cation was encountered.
3. Summary
However, the fact that sodium atoms in a vapour lamp have an excitation from the 3s to the 3p orbital and the subsequent reverse transition leads to the emission does not mean that the excitation must have been an electron from the 3s orbital of a ground-state uncharged sodium atom to its 3p orbital.
1. Both an uncharged sodium atom and a sodium cation will give a yellow colour in a flame test
The selection rules for symmetry-allowed and symmetry-forbidden transitions in electronic spectroscopy are determined by the transition moment integral:
Specifically, if the transition moment function,
In other words, quantum mechanics dictates that transitions s to s, p to p, d to d, and f to f, are all forbidden on symmetry grounds in a spherical species. An excitation of an electron in a 2p orbital in a sodium cation is thus most likely to be 2p to 3s or 2p to 3d. An excitation from an already-excited sodium cation with a 3s electron is most likely to be 3s to 3p. The only possible relaxation pathway for an excited state sodium cation with a 3p electron is 3p to 3s unless there is (somehow) a vacancy in a 2s or 1s orbital. An excited state sodium cation with a 3d electron can relax 3d to 3p or 3d to 2p but not 3d to 3s.
In reality, spin-forbidden transitions are not necessarily impossible because vibrational coupling can alter the symmetry sufficiently for them to become weakly allowed, but that is not important in this case. Also, since these selection rules apply equally to a sodium atom as they do to a sodium cation, nothing in the above prevents uncharged sodium atoms present in a flame from producing a yellow colour - in fact, if uncharged sodium atoms are present, they will produce a colour. What the rules do explain is why sodium cations will also produce a yellow colour if present in a flame - though there is no 3s electron in the ground state, that does not mean that excitations can't be 2p to 3s to 3p and reverse, to give the flame colour, with an alternative being a 2p to 3d excitation with a 3d to 3p to 3s to 2p relaxation pathway.
2. Could we have uncharged sodium atoms in a flame?
If there is sodium vapour around, as in a sodium vapour lamp, sure. If there is sodium metal around, also sure - metallic sodium melts at 98 degC and boils 883 degC, so a bunsen is easily hot enough to turn Na(s) into Na(g). However, sodium metal oxidises rapidly in air and sodium oxide (Na2O) melts at 1132 degC and sublimes around 1275 degC. Even if sodium metal is present, placing it into a flame with an ample supply of air guarantees it will become sodium ions as part of an oxide rapidly.
For a flame test with sodium chloride, the question that @dumNerd asks is, for me, critical... if sodium chloride were somehow producing uncharged sodium atoms in a flame test, where is the electron coming from to reduce the sodium cation?
2 Na+ + 2 Cl- -----> 2 Na + Cl2 might seem plausible at first, but:
- the reaction as written has an E0 ~ -4 V and so would require very forcing conditions
- the reaction is highly endothermic, deltaH ~ +820 kJ mol-1
- chlorine gas is pretty noticeable
- sodium metal burns in a chlorine environment to make NaCl, so why would we expect the reverse to happen in a flame?
- sodium chloride is a highly stable ionic substance, melting point 800 degC, boiling point 1465 degC
In short, the sodium cation is much more stable than sodium metal because the latter is easily oxidised to the former, even in air at room temperature. In a high-energy environment like a flame, short-lived and unstable species can be formed but it is difficult to see how or why sodium cations would be reduced unless something even more readily oxidised were present - and any such would surely be oxidised by air long before a sodium cation was encountered.
3. Summary
- Though the selection rules and related mathematics are well beyond HSC level, the conclusion they support is not too complicated. Whether it be an excited state sodium cation or uncharged sodium atom, once an electron is excited into the 3p subshell, its only (reasonable) relaxation pathway is via the emission of yellow light whose wavelength corresponds to the gap between a 3p and the 3s orbital.
- Explaining how a flame test using a sodium compound can come to introduce sodium cations into a flame is straight-forward. It is difficult, however, to conjecture a plausible chemical explanation for those cations being reduced to produce uncharged atoms of sodium metal in a flame.
- Personal Recollection: I recall doing experimental work in my undergraduate chemistry studies of the spectroscopy of flames, looking for evidence about the short-lived intermediates from combustion that can be detected in that environment. The molecule C2, for example, can be detected easily and its spectroscopic properties used to calculate its bond strength and bond order. I remember my surprise that the two most intense transitions we detected in the blue flame of an ordinary bunsen burner were the D lines of sodium. The lecturer in charge explained that these are caused by salt in the air that deposits onto the inner walls of the bunsen burner itself. The lines have such great intensity that they are observable even when only a tiny amount of sodium is present, and I learned from it that salt contamination is something to bear in mind in some types of spectroscopy.


