-
Looking for HSC notes and resources? Check out our Notes & Resources page
Make a Difference – Donate to Bored of Studies!
Students helping students, join us in improving Bored of Studies by donating and supporting future students!
can someone please explain complexation (1 Viewer)
- Thread starter SadCeliac
- Start date
STBAccuracy
Member
I found this in the Pearson Chem textbook (dm me if you need a copy):
Transition metal ions are small and highly charged. This high charged density results in an ability to strongly attract anions/small polar molecules (ligands), forming a complex ion.
The process of ligands forming an ion/compound around a metal ion is referred to complexation.
Below is an example of a metal complex Al(OH)4-
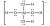
The charge of the metal complex can be found by determining the oxidation state of the total compound.
There wasn't much else I could find on complexation for the scope of the syllabus, but I hope it helped!
Transition metal ions are small and highly charged. This high charged density results in an ability to strongly attract anions/small polar molecules (ligands), forming a complex ion.
The process of ligands forming an ion/compound around a metal ion is referred to complexation.
Below is an example of a metal complex Al(OH)4-

The charge of the metal complex can be found by determining the oxidation state of the total compound.
- Aluminium has a charge +3
- Hydroxide has a charge -1
- Thus net charge = 3 + (-1) * 4 = -1
There wasn't much else I could find on complexation for the scope of the syllabus, but I hope it helped!
tysmI found this in the Pearson Chem textbook (dm me if you need a copy):
Transition metal ions are small and highly charged. This high charged density results in an ability to strongly attract anions/small polar molecules (ligands), forming a complex ion.
The process of ligands forming an ion/compound around a metal ion is referred to complexation.
Below is an example of a metal complex Al(OH)4-
View attachment 40021
The charge of the metal complex can be found by determining the oxidation state of the total compound.
- Aluminium has a charge +3
- Hydroxide has a charge -1
- Thus net charge = 3 + (-1) * 4 = -1
There wasn't much else I could find on complexation for the scope of the syllabus, but I hope it helped!
what other examples do we need to know?
STBAccuracy
Member
The syllabus states we need to know complexation reactions to determine the presence of 8 cations.tysm
what other examples do we need to know?
barium (Ba2+), calcium (Ca2+), magnesium (Mg2+), lead(II) (Pb2+), silver ion (Ag+), copper(II) (Cu2+), iron(II) (Fe2+), iron(III) (Fe3+)
The one that I remember is the Iron (III) thiocyanate complex (Blood red) since we just used precipitate reactions and flame tests to test the other compounds.
Below is basically how we learnt how to discern all the cations.
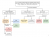
The crucial point about complexation is that these species are formed and held together by covalent bonds between the metal centre and each ligand. They are almost always coordinate covalent bonds, whereby the ligand donates a lone pair of electrons to form the covalent bond, rather than by one electron coming from the ligand and one from the metal.
This sort of bonding to form coordination complexes occurs extensively for metals, whether it be by:
Complexation can have significant impacts on solubility. For example, silver(I) chloride re-dissolves when ammonia is added as the formation of the diamminesilver(I) cation:
decreases the concentration of unbound Ag+ cations in the solution, thereby drawing the solubility equilibrium:
to the right, according to Le Chatelier's Principle.
Similarly, aluminium(III) hydroxide is amphoteric as it reactions with acid (obviously) but also with base to form the tetrahydroxyaluminate(III) anion:
which also explains why insoluble aluminium hydroxide dissolves in a concentration sodium hydroxide solution.
This sort of bonding to form coordination complexes occurs extensively for metals, whether it be by:
- as hydration shells around cations (like in [Fe(H2O)6]3+, the hexaquairon(III) cation), or
- the blood-red thiocyanatoiron(III) cation FeSCN2+ (more formally, the pentaaquathiocyanatoiron(III) cation [Fe(H2O)5SCN]3+) which actually has the covalent bond from the Fe to the N atom, or
- in biological system likes the binding of oxygen to the iron centre of haemoglobin.
Complexation can have significant impacts on solubility. For example, silver(I) chloride re-dissolves when ammonia is added as the formation of the diamminesilver(I) cation:
Ag+ (aq) + 2 NH3 (aq) <---> [Ag(NH3)3]+
decreases the concentration of unbound Ag+ cations in the solution, thereby drawing the solubility equilibrium:
AgCl (s) <----> Ag+ (aq) + Cl- (aq)
to the right, according to Le Chatelier's Principle.
Similarly, aluminium(III) hydroxide is amphoteric as it reactions with acid (obviously) but also with base to form the tetrahydroxyaluminate(III) anion:
Al(OH)3 (s) + OH- (aq) <----> [Al(OH)4]- (aq)
which also explains why insoluble aluminium hydroxide dissolves in a concentration sodium hydroxide solution.
wizzkids
Well-Known Member
- Joined
- Jul 13, 2016
- Messages
- 366
- Gender
- Undisclosed
- HSC
- 1998
Yes, there is a lot of jargon around this topic. Complexation, as far as the HSC Chemistry course is concerned, refers to certain metallic cations that can become surrounded by a shell containing 4 or 6 negatively charged anions, or polar molecules with their delta-negative bits pointing towards the central cation. This is a reversible equilibrium reaction, therefore it has an equilibrium constant. The concentration of the anions has to reach a critical level before an appreciable amount of the complexed ion starts to form.
These complexed ions are sometimes intensely coloured. This is because the bonding electrons have energy transitions around 2 to 3 electron-Volts which is slap-bang in the middle of the visible light spectrum. Complexation can be used to detect the presence of particular metallic cations, as others have said.
These complexed ions are sometimes intensely coloured. This is because the bonding electrons have energy transitions around 2 to 3 electron-Volts which is slap-bang in the middle of the visible light spectrum. Complexation can be used to detect the presence of particular metallic cations, as others have said.
Note that complexes of silver typically have two ligands, like:
diamminesilver(I) cation = [Ag(NH3)2]+
dicyanoargentate(I) anion = [Ag(CN)2]-
For most HSC examples, the equilibrium constants for their formation are large and so complexation is strongly favoured. For example, Q31 of the 2022 HSC involved the formation of the diamminesilver(I) cation, where the equilibrium
was stated to have an equilibrium constant of 1.6 x 107 at room temperature.
diamminesilver(I) cation = [Ag(NH3)2]+
dicyanoargentate(I) anion = [Ag(CN)2]-
For most HSC examples, the equilibrium constants for their formation are large and so complexation is strongly favoured. For example, Q31 of the 2022 HSC involved the formation of the diamminesilver(I) cation, where the equilibrium
Ag+ (aq) + 2 NH3 (aq) <----> [Ag(NH3)2]+ (aq)
was stated to have an equilibrium constant of 1.6 x 107 at room temperature.
