-
Looking for HSC notes and resources? Check out our Notes & Resources page
Make a Difference – Donate to Bored of Studies!
Students helping students, join us in improving Bored of Studies by donating and supporting future students!
Davisson germer experiment (1 Viewer)
- Thread starter akkjen
- Start date
ABBAS38
Member
- Joined
- Aug 16, 2019
- Messages
- 81
- Gender
- Male
- HSC
- 2019
hi, can someone please explain the davisson and germer experiment to me and how it aided debroglie, i can't find any resources that are high school level and the easier ones dont make sense to me . thanks in advance!!!
yes, i watched that he had a little bit on the actual experiment at the end. i didn't understand how it related to debroglie
Balajanovski
Member
- Joined
- Jun 17, 2017
- Messages
- 67
- Gender
- Male
- HSC
- 2019
Apologies if the explanation is a bit convoluted. I'm about to go to sleep.
The Davisson and Germer experiment was, pretty much Thomas Young's double slit experiment for electrons.
It involved shining a beam of electrons at a nickel crystal lattice.
What they surprisingly observed is an interference pattern on the detector, much like the one in Young's double slit experiment (Added detail: The detector would be moved about to measure the current from incident scattered electrons, which was analogous to the intensity of a wave).
This interference pattern involved a maximum intensity of electrons at angle zero, followed by a minimum, and then a lesser maximum.

Interference is a property exclusive to waves, meaning that electrons could exhibit wave behaviour. This supported de Broglie's hypothesis on the existence of matter waves. (de Broglie's hypothesis suggests that matter may exhibit both wave and particle properties).
The reason why the electrons behaved like waves to produce an interference pattern in the nickel crystal is because the lattice of the nickel crystal acted like a diffraction grating. Electrons would pass through the minute "slits" between the uniformly spaced nickel atoms, to diffract like waves (Recall that wave diffraction occurs when the slit a wave passes through is less than or approximately equal to the wavelength).
Added detail:
The equation used to predict this interference pattern is not the standard diffraction grating equation. This is because when the electrons pass between the atoms, they are then reflected back out to pass through the nickel "diffraction grating" a second time. The equation, for your interest, is called Bragg's law.
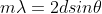
Notice how it is very similar to the diffraction grating equation, with the extra 2 constant.

The Davisson and Germer experiment was, pretty much Thomas Young's double slit experiment for electrons.
It involved shining a beam of electrons at a nickel crystal lattice.
What they surprisingly observed is an interference pattern on the detector, much like the one in Young's double slit experiment (Added detail: The detector would be moved about to measure the current from incident scattered electrons, which was analogous to the intensity of a wave).
This interference pattern involved a maximum intensity of electrons at angle zero, followed by a minimum, and then a lesser maximum.
Interference is a property exclusive to waves, meaning that electrons could exhibit wave behaviour. This supported de Broglie's hypothesis on the existence of matter waves. (de Broglie's hypothesis suggests that matter may exhibit both wave and particle properties).
The reason why the electrons behaved like waves to produce an interference pattern in the nickel crystal is because the lattice of the nickel crystal acted like a diffraction grating. Electrons would pass through the minute "slits" between the uniformly spaced nickel atoms, to diffract like waves (Recall that wave diffraction occurs when the slit a wave passes through is less than or approximately equal to the wavelength).
Added detail:
The equation used to predict this interference pattern is not the standard diffraction grating equation. This is because when the electrons pass between the atoms, they are then reflected back out to pass through the nickel "diffraction grating" a second time. The equation, for your interest, is called Bragg's law.
Notice how it is very similar to the diffraction grating equation, with the extra 2 constant.

i havent read it yet but it looks greatApologies if the explanation is a bit convoluted. I'm about to go to sleep.
The Davisson and Germer experiment was, pretty much Thomas Young's double slit experiment for electrons.
It involved shining a beam of electrons at a nickel crystal lattice.
What they surprisingly observed is an interference pattern on the detector, much like the one in Young's double slit experiment (Added detail: The detector would be moved about to measure the current from incident scattered electrons, which was analogous to the intensity of a wave).
This interference pattern involved a maximum intensity of electrons at angle zero, followed by a minimum, and then a lesser maximum.
Interference is a property exclusive to waves, meaning that electrons could exhibit wave behaviour. This supported de Broglie's hypothesis on the existence of matter waves. (de Broglie's hypothesis suggests that matter may exhibit both wave and particle properties).
The reason why the electrons behaved like waves to produce an interference pattern in the nickel crystal is because the lattice of the nickel crystal acted like a diffraction grating. Electrons would pass through the minute "slits" between the uniformly spaced nickel atoms, to diffract like waves (Recall that wave diffraction occurs when the slit a wave passes through is less than or approximately equal to the wavelength).
Added detail:
The equation used to predict this interference pattern is not the standard diffraction grating equation. This is because when the electrons pass between the atoms, they are then reflected back out to pass through the nickel "diffraction grating" a second time. The equation, for your interest, is called Bragg's law.
Notice how it is very similar to the diffraction grating equation, with the extra 2 constant.

thanks for taking the time to help me out!!!!!!!!
Balajanovski
Member
- Joined
- Jun 17, 2017
- Messages
- 67
- Gender
- Male
- HSC
- 2019
No worries. Just edited it to add an extra illustration so remember to refresh the page.i havent read it yet but it looks great
thanks for taking the time to help me out!!!!!!!!
Arrowshaft
Well-Known Member
Ah yes Bragg’s equation, just as a side note; I’d also mention the significance of the 54V potential difference and the contribution of G.P. Thomson. Very comprehensive response though!Apologies if the explanation is a bit convoluted. I'm about to go to sleep.
The Davisson and Germer experiment was, pretty much Thomas Young's double slit experiment for electrons.
It involved shining a beam of electrons at a nickel crystal lattice.
What they surprisingly observed is an interference pattern on the detector, much like the one in Young's double slit experiment (Added detail: The detector would be moved about to measure the current from incident scattered electrons, which was analogous to the intensity of a wave).
This interference pattern involved a maximum intensity of electrons at angle zero, followed by a minimum, and then a lesser maximum.

Interference is a property exclusive to waves, meaning that electrons could exhibit wave behaviour. This supported de Broglie's hypothesis on the existence of matter waves. (de Broglie's hypothesis suggests that matter may exhibit both wave and particle properties).
The reason why the electrons behaved like waves to produce an interference pattern in the nickel crystal is because the lattice of the nickel crystal acted like a diffraction grating. Electrons would pass through the minute "slits" between the uniformly spaced nickel atoms, to diffract like waves (Recall that wave diffraction occurs when the slit a wave passes through is less than or approximately equal to the wavelength).
Added detail:
The equation used to predict this interference pattern is not the standard diffraction grating equation. This is because when the electrons pass between the atoms, they are then reflected back out to pass through the nickel "diffraction grating" a second time. The equation, for your interest, is called Bragg's law.
Notice how it is very similar to the diffraction grating equation, with the extra 2 constant.

dude, is this above the syllabus again? i havent learnt about G.P. Thomson. also what is the 54V?Ah yes Bragg’s equation, just as a side note; I’d also mention the significance of the 54V potential difference and the contribution of G.P. Thomson. Very comprehensive response though!
i think it is? we didn't learn that in class either.dude, is this above the syllabus again? i havent learnt about G.P. Thomson. also what is the 54V?
Arrowshaft
Well-Known Member
Davisson-Germer is in the syllabus, however G.P. Thomson isn’t, just thought it’d be nice to share some extra info. 
what about bragg's law?Davisson-Germer is in the syllabus, however G.P. Thomson isn’t, just thought it’d be nice to share some extra info.
Arrowshaft
Well-Known Member
Bragg’s Law was a part of the old syllabus, however my tutor advised me its good to know and apply nevertheless as it is a DIRECT validation of the theory. Since the calculations performed using Bragg’s Lawwhat about bragg's law?
yields the same answer as using de Broglie’s derivation,
sorry for the bother, but can you give me a dumbed down explanation of thisBragg’s Law was a part of the old syllabus, however my tutor advised me its good to know and apply nevertheless as it is a DIRECT validation of the theory. Since the calculations performed using Bragg’s Law
yields the same answer as using de Broglie’s derivation,
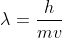
thanks in advance
Arrowshaft
Well-Known Member
Bragg’s law essentially just calculated the wavelength of the electrons by determining the interference patterns of it through the nickel crystal. Now, as Balajanovski said, they pass through twice through the crystal so it’s not your typical diffraction equation, rather multiplied by 2. By analysing the maxima and minima of the interference patterns produced, we can determine the wavelength of the electron (similar to how in school you would’ve done an experiment to measure the bright spots from the double slit experiment and using the size of the slits to find out the wavelength of light). The reason they undergo diffraction in the first place is because the gaps between the nickel atoms are small enough to act as those slits that can cause the diffraction.sorry for the bother, but can you give me a dumbed down explanation of this
thanks in advance
Davisson-Germer is not in the syllabus.....Davisson-Germer is in the syllabus, however G.P. Thomson isn’t, just thought it’d be nice to share some extra info.
Arrowshaft
Well-Known Member
“investigate de Broglie’s matter waves, and the experimental evidence that developed the following formula...”Davisson-Germer is not in the syllabus.....
It doesn’t explicitly state Davisson-Germer, but I guess you could also use other scientists, maybe GP as well
I am under the impression that is simply referring to a basic understanding of electron stability as it is a minor dot point. Whilst I do know the experiment, I would argue that one would be best focusing on other areas directly in the syllabus the day before the exam!“investigate de Broglie’s matter waves, and the experimental evidence that developed the following formula...”
It doesn’t explicitly state Davisson-Germer, but I guess you could also use other scientists, maybe GP as well
