Yeah I was going to start the physics one. But if it's drag n drop not just the quiz but the practice trial it might not be worth doing?Yeah, Learnable is a bit dodgy and I can see why the exam isn't too well made. I think it'd be better to just be doing past trials, hsc exams and the like.
-
Looking for HSC notes and resources? Check out our Notes & Resources page
Make a Difference – Donate to Bored of Studies!
Students helping students, join us in improving Bored of Studies by donating and supporting future students!
HSC Chemistry SOLUTIONS / Thoughts / Predictions (1 Viewer)
- Thread starter jazz519
- Start date
Speed’o’sound Sonic
Well-Known Member
- Joined
- Nov 4, 2019
- Messages
- 495
- Gender
- Male
- HSC
- 2020
Honesty, I’m not gonna sit the physics one after the state of the Chem one todayYeah I was going to start the physics one. But if it's drag n drop not just the quiz but the practice trial it might not be worth doing?
Yeah I have had a brief look at the exams as well. They are okay but in terms of preparing you for HSC style questions not so much as you can't just drag and drop in the HSC.I just tried sitting the chemistry one - I don't really recommend it:
- wanted us to explain the NMR spectrum of aspirin (which is interesting, to say the least)
- all the extended responses are drag and drop, which makes it completely not worth doing
- multiple questions had incomplete stimulus data (e.g. a Hess' Law question was missing one of the molar enthalpy of formatoin)
- the typed number answers were worth 2-3 marks and you could only enter a single number, and there was no indication of how precise they wanted
- scrolling up and down to see the stimulus was a pain
Bit hard to explain without just showing the questions, but it was extremely wack.
The questions are not too complicated. If you have a good approach and spend time on a couple of questions you will see they become very repetitiveOh God.. at most I know how the properties and uses for each but its literally a TRAVESTY when the question asks to draw polymers out and your given two reactants. omg and I suck at
Addition polymers example:

1. Move all bonds not involved in double bond to the vertical position
2. Redraw the structure 3 times without double bond
3. Connect those 3 repeats together
Condensation polymers example:
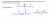
This is the other way around but if you want to go from the two reactants to polymer
1. Remove 1 water from inside of the monomers and 1 water from outside of the monomers
2. Join together and put bracket and n
Unfortunately the connection between structure, property and uses are problematic. For some polymers such as nylon, there is no generally agreed connection. It is hydrophilic but nylon is used because of its strength not ability to absorb water. There is no way to justify how it is stronger than other polymers based on structure, however.Oh God.. at most I know how the properties and uses for each but its literally a TRAVESTY when the question asks to draw polymers out and your given two reactants. omg and I suck at
Speed’o’sound Sonic
Well-Known Member
- Joined
- Nov 4, 2019
- Messages
- 495
- Gender
- Male
- HSC
- 2020
Thankyou!!! Chemistry godThe questions are not too complicated. If you have a good approach and spend time on a couple of questions you will see they become very repetitive
Addition polymers example:
View attachment 29354
1. Move all bonds not involved in double bond to the vertical position
2. Redraw the structure 3 times without double bond
3. Connect those 3 repeats together
Condensation polymers example:
View attachment 29355
This is the other way around but if you want to go from the two reactants to polymer
1. Remove 1 water from inside of the monomers and 1 water from outside of the monomers
2. Join together and put bracket and n
bekind2003
New Member
- Joined
- Aug 9, 2020
- Messages
- 20
- Gender
- Female
- HSC
- 2020
omg hold up the steps for drawing addition polymers is so helpful thank you! Could you clarify the condensation polymers again? So when were drawing what is the water outside of the monomers? (sorry I'm slow)The questions are not too complicated. If you have a good approach and spend time on a couple of questions you will see they become very repetitive
Addition polymers example:
View attachment 29354
1. Move all bonds not involved in double bond to the vertical position
2. Redraw the structure 3 times without double bond
3. Connect those 3 repeats together
Condensation polymers example:
View attachment 29355
This is the other way around but if you want to go from the two reactants to polymer
1. Remove 1 water from inside of the monomers and 1 water from outside of the monomers
2. Join together and put bracket and n
bekind2003
New Member
- Joined
- Aug 9, 2020
- Messages
- 20
- Gender
- Female
- HSC
- 2020
ahh yeah its pretty useless then, but there's always a sneaky question about polymer properties in most trials which is annoyingUnfortunately the connection between structure, property and uses are problematic. For some polymers such as nylon, there is no generally agreed connection. It is hydrophilic but nylon is used because of its strength not ability to absorb water. There is no way to justify how it is stronger than other polymers based on structure, however.
A condensation polymer forms via water being eliminatedomg hold up the steps for drawing addition polymers is so helpful thank you! Could you clarify the condensation polymers again? So when were drawing what is the water outside of the monomers? (sorry I'm slow)
This means if you want to draw from 2 monomers to a polymer we have to remove the water
Basically take off the OH on the left structure on both sides
Take off the H from the NH2 on the right structure on both sides
That in total means you are removing 2OH and 2H which is 2H2O
bekind2003
New Member
- Joined
- Aug 9, 2020
- Messages
- 20
- Gender
- Female
- HSC
- 2020
ohh omg THANKYOU legendA condensation polymer forms via water being eliminated
This means if you want to draw from 2 monomers to a polymer we have to remove the water
Basically take off the OH on the left structure on both sides
Take off the H from the NH2 on the right structure on both sides
That in total means you are removing 2OH and 2H which is 2H2O
I don't remember studying any of those methods in my class. Plus for all the trial and hsc exam papers I have done, I don't remember seeing any questions on those methods.Guys the syllabus says only precipitation titrations. Do we need to know Mohrs, Volhard's and Fajans method? If not can someone please list all the important titrations?
MrGresh
Active Member
Yeah we didnt do them either. Many exams include them, but walk you through them such that you do not need to actually know the methods beforehand.I don't remember studying any of those methods in my class. Plus for all the trial and hsc exam papers I have done, I don't remember seeing any questions on those methods.
I don't think it's in NESA's interests to include these titrations as it is essentially luck whether students know the methods. This being said, I think it's a safe choice to be familiar with them at least in terms of calculation.
We will see!
MrGresh
Active Member
Idk I've seen it done differently in different places. I'd go with NESA for obvious reasonsHi lads and lasses,
when rounding up pH and pOH do u recommend rounding up to sig figs or decimal places? Because for the whole year my teacher has been telling us to round it up to one decimal places, but hsc Sample solutions suggest otherwise
Speed’o’sound Sonic
Well-Known Member
- Joined
- Nov 4, 2019
- Messages
- 495
- Gender
- Male
- HSC
- 2020
Let’s gooo lads. Final exam preparation, now that physics is out of the way it’s Chem Chem Chem!  how are we all feeling so far? How’s everyone doing
how are we all feeling so far? How’s everyone doing
Speed’o’sound Sonic
Well-Known Member
- Joined
- Nov 4, 2019
- Messages
- 495
- Gender
- Male
- HSC
- 2020
It’s you againIt's the race to the end!
Revising hydrocarbons though
yeah early mod 7 is a bit of a weak spot for me too. Obviously mod5 and 6 are breezy but 7 and 8 are a bit harder
You've been here for all of them too!It’s you againI swear you’ve been on all of them, maths ext2 and physics as well?
yeah early mod 7 is a bit of a weak spot for me too. Obviously mod5 and 6 are breezy but 7 and 8 are a bit harder
Speed’o’sound Sonic
Well-Known Member
- Joined
- Nov 4, 2019
- Messages
- 495
- Gender
- Male
- HSC
- 2020
Yep! Mad respect man. What course u going for?You've been here for all of them too!
Engi type stuffYep! Mad respect man. What course u going for?
But I'm indecisive, so that might change.
