-
YOU can help the next generation of students in the community! Share your trial papers and notes on our Notes & Resources page
Make a Difference – Donate to Bored of Studies!
Students helping students, join us in improving Bored of Studies by donating and supporting future students!
Multiple Choice (1 Viewer)
- Thread starter Rafy
- Start date
Dude.. I've uploaded them in a thread below. Have a look.Can some one please upload the mc questions.
PLEASE PLEASE PLEASE!!
CalSandercock
New Member
- Joined
- Jul 9, 2011
- Messages
- 7
- Gender
- Male
- HSC
- 2011
Hey about the 15 question. If you think about the acids and their pHs if the strong acid has a pH of 1 and the weak acid has a pH of 4 or something and they both are 0.100/mol , then doesn't it make sense that there are is a smaller concentration of hydrogen ions in the weak acid and would therefore take less base to neutralise?
Just sayin'
Just sayin'
but it was worded properly, you just misread =/
'...400g of charcoal is reacted, assuming equal amounts are consumed in each reaction'
That is very easy to misinterpret as 400 g is reacted equally for each reaction, but it really wanted 400 g reacted equally BETWEEN each reaction. Its just bad wording... stupid BOS :/
Tasha270494
Member
- Joined
- Jan 13, 2010
- Messages
- 58
- Gender
- Female
- HSC
- 2011
YEP. Everyone got Q.12 wrong - it is in fact BI'm just curious, for question 12 it says 'electrochemical activity' does that mean that it's from positive to negative, negative to positive, or actually the largest MAGNITUDE to the lowest... i.e. B
Electrochemical activity includes both the ability to oxidise or reduce and therefore you havto take the absolute value and then rank it.
BadMeetsEvil
Member
- Joined
- Oct 28, 2011
- Messages
- 162
- Gender
- Male
- HSC
- N/A
nah it should be from negative to positiveYEP. Everyone got Q.12 wrong - it is in fact B
Electrochemical activity includes both the ability to oxidise or reduce and therefore you havto take the absolute value and then rank it.
http://www.chemguide.co.uk/physical/redoxeqia/ecs.html
Tasha270494
Member
- Joined
- Jan 13, 2010
- Messages
- 58
- Gender
- Female
- HSC
- 2011
?nah it should be from negative to positive
http://www.chemguide.co.uk/physical/redoxeqia/ecs.html
I'm pretty sure it is B (even though I put C)
You can see that Pd wants to give away electrons (reduce) moreso than Cd2+ gaining electrons (oxidise) since it requires a higher voltage - more likely to occur.
sonispucca
New Member
- Joined
- Nov 10, 2009
- Messages
- 28
- Gender
- Female
- HSC
- 2011
Me too! The two I got wrong were both ones where I was considering changing my answer and then ran out of time so i didnt. Aaarghgh.I believe you are all correct indeed =]
i got 18/20 freaking silly mistakes! didnt have time to go back and check =[[
BadMeetsEvil
Member
- Joined
- Oct 28, 2011
- Messages
- 162
- Gender
- Male
- HSC
- N/A
reduce- give away electrons? Oxidise- gain electrons??
I'm pretty sure it is B (even though I put C)
You can see that Pd wants to give away electrons (reduce) moreso than Cd2+ gaining electrons (oxidise) since it requires a higher voltage - more likely to occur.
c'mon. At least brush up on your basic informations before making an argument
Tasha270494
Member
- Joined
- Jan 13, 2010
- Messages
- 58
- Gender
- Female
- HSC
- 2011
... you know what I meant.reduce- give away electrons? Oxidise- gain electrons?
c'mon. At least brush up on your basic informations before making an argument
Anyways, its okay if you don't agree
Impossibru!! Never heard of such a preposterous claim about "absolute values" in the 4-5 different sets of chem notes/books i have so... yea.. I am quite confident "electrochemical activity" simply is BOS's convoluted way of saying "Reactivity" in which case, D would be correct.YEP. Everyone got Q.12 wrong - it is in fact B
Electrochemical activity includes both the ability to oxidise or reduce and therefore you havto take the absolute value and then rank it.
Tasha270494
Member
- Joined
- Jan 13, 2010
- Messages
- 58
- Gender
- Female
- HSC
- 2011
.. never saw it in textbooks either. But activity series is different to electrochemical series:Impossibru!! Never heard of such a preposterous claim about "absolute values" in the 4-5 different sets of chem notes/books i have so... yea.. I am quite confident "electrochemical activity" simply is BOS's convoluted way of saying "Reactivity" in which case, D would be correct.
"E° values give you a way of comparing the positions of equilibrium when these elements lose electrons to form ions in solution.
The more negative the E° value, the further the equilibrium lies to the left - the more readily the element loses electrons and forms ions.
The more positive (or less negative) the E° value, the further the equilibrium lies to the right - the less readily the element loses electrons and forms ions."
http://www.chemguide.co.uk/physical/redoxeqia/introduction.html#top
Last edited:
BadMeetsEvil
Member
- Joined
- Oct 28, 2011
- Messages
- 162
- Gender
- Male
- HSC
- N/A
lol wth. You're contradicting yourself... never saw it in textbooks either. But activity series is different to electrochemical series:
"E° values give you a way of comparing the positions of equilibrium when these elements lose electrons to form ions in solution.
The more negative the E° value, the further the equilibrium lies to the left - the more readily the element loses electrons and forms ions.
The more positive (or less negative) the E° value, the further the equilibrium lies to the right - the less readily the element loses electrons and forms ions."
http://www.chemguide.co.uk/physical/redoxeqia/introduction.html#top
That extract supports reactivity series is the same as electrochemical activity. Therefore the more negative, the more it is likely to lose electron thus it has higher electrochemical activity. Which is pretty much the same definition as a reactivity series.
Tasha270494
Member
- Joined
- Jan 13, 2010
- Messages
- 58
- Gender
- Female
- HSC
- 2011
lol. This is harder to explain than I thought..lol wth. You're contradicting yourself.
That extract supports reactivity series is the same as electrochemical activity. Therefore the more negative, the more it is likely to lose electron thus it has higher electrochemical activity. Which is pretty much the same definition as a reactivity series.
Electrochemical activity refers to the oxidising/reducing power.
Activity series is only metals and thus your only taking into consideration the ability for it to oxidise and lose electrons.
Look at the image:
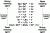
Well quoting from here http://www.saskschools.ca/curr_content/chem30_05/6_redox/redox2_5.htm
An activity series list metals and various other elements in order of their reactivity, with the most reactive elements at the top and the least reactive of the series at the bottom. For any two metals, the metal listed higher in the table is the most readily oxidized.
Notice the word oxidised, so it doesnt take the absolute value...
Edit: Ok look, i really dont know anymore and dont even know what im saying/what my argument is ^^
An activity series list metals and various other elements in order of their reactivity, with the most reactive elements at the top and the least reactive of the series at the bottom. For any two metals, the metal listed higher in the table is the most readily oxidized.
Notice the word oxidised, so it doesnt take the absolute value...
Edit: Ok look, i really dont know anymore and dont even know what im saying/what my argument is ^^
Last edited:
K
khorne
Guest
Li > K > Sr > Ca > Na > Mg > Al > Zn > Cr > Fe > Cd > Co > Ni > Sn > Pb > H > Cu > Ag > Hg > Pt > AuWell quoting from here http://www.saskschools.ca/curr_content/chem30_05/6_redox/redox2_5.htm
An activity series list metals and various other elements in order of their reactivity, with the most reactive elements at the top and the least reactive of the series at the bottom. For any two metals, the metal listed higher in the table is the most readily oxidized.
Notice the word oxidised, so it doesnt take the absolute value...
Thats from wiki...notice all the samples were METALS.
