-
Best of luck to the class of 2025 for their HSC exams. You got this! Let us know your thoughts on the HSC exams here
Make a Difference – Donate to Bored of Studies!
Students helping students, join us in improving Bored of Studies by donating and supporting future students!
Need some help with chem q's (1 Viewer)
- Thread starter hs17
- Start date
Question 18
Since the combustion heated 200.0 g of water by 19.9 oC, we know that
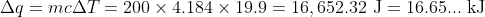
We also know that the heat of combustion is 3329 kJ mol-1 which means that the enthalpy of combustion is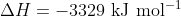 . From this, we can determine the chemical amount of fuel burned, and thus its molar mass:
. From this, we can determine the chemical amount of fuel burned, and thus its molar mass:
}} \times \text{n(fuel in equation)} \\ -3329 &= \cfrac{-16.65...}{\text{n(fuel burned)}} \times 1 \qquad \text{as the coefficient of the fuel is 1 in this enthalpy of combustion equation} \\ \text{n(fuel burned)} &= \cfrac{16.65...}{3329} \\ \text{M(fuel)} &= \cfrac{m}{n} \\ &= 0.44 \div \cfrac{16.65...}{3329} \\ &= 87.96...\ \text{g mol}^{-1} \end{align*})
And, since
I think the answer is C.
EDIT: I just noticed the table in the question! Matching the calculated molar mass of 88.0 to the table, the answer is still 1-pentanol and so is still C! However, it would be D, matching the answer quoted by the OP, if the answers were meant to be in the same order as the table, and thus have 1-pentanol last.
Since the combustion heated 200.0 g of water by 19.9 oC, we know that
We also know that the heat of combustion is 3329 kJ mol-1 which means that the enthalpy of combustion is
And, since
- 1-butanol is CH3CH2CH2CH2OH, with M(1-butanol)
---> NOT "A," does not match the calculated M(fuel).
- butane is CH3CH2CH2CH3, with M(butane)
---> NOT "B," does not match the calculated M(fuel).
- 1-pentanol is CH3CH2CH2CH2CH2OH with M(1-pentanol)
---> "C," does match the calculated M(fuel).
- pentane is CH3CH2CH2CH2CH3, with M(pentane)
---> NOT "D," does not match the calculated M(fuel).
I think the answer is C.
EDIT: I just noticed the table in the question! Matching the calculated molar mass of 88.0 to the table, the answer is still 1-pentanol and so is still C! However, it would be D, matching the answer quoted by the OP, if the answers were meant to be in the same order as the table, and thus have 1-pentanol last.
Last edited:
Question 15:
pKa(ethanoic acid) = 4.76 at 25 oC
So, pKb(ethanoate ion) = pKw - pKa(ethanoic acid) = 14.00 - 4.76 = 9.24
Constructing the usual ICE table with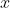 being the [OH-] at equilibrium, you should get
being the [OH-] at equilibrium, you should get
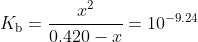
The equilibrium clearly lies to the left with such a small equilibrium constant, so the assumption that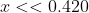 and thus that
and thus that 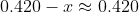 is justified, and so:
is justified, and so:
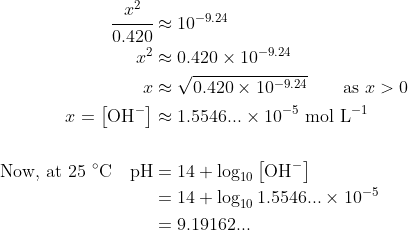
The answer is C.
pKa(ethanoic acid) = 4.76 at 25 oC
So, pKb(ethanoate ion) = pKw - pKa(ethanoic acid) = 14.00 - 4.76 = 9.24
Constructing the usual ICE table with
The equilibrium clearly lies to the left with such a small equilibrium constant, so the assumption that
The answer is C.
Question 14:
The overall equation for the use of glucose requires combining reactions 2 and 3 to eliminate the intermediate (ethanol). When this is done, you see that the overall equation is reaction 1 backwards, so the CO2 produced in reactions 2 and 3 exactly matches the CO2 consumed in reaction 1. Hence, the answer is (C).
The overall equation for the use of glucose requires combining reactions 2 and 3 to eliminate the intermediate (ethanol). When this is done, you see that the overall equation is reaction 1 backwards, so the CO2 produced in reactions 2 and 3 exactly matches the CO2 consumed in reaction 1. Hence, the answer is (C).
The answer is (A) - Add hydrochloric acid, then ammoniaHivaclibtibcharkwa said:View attachment 32804@jazz519 bless me with your detailed answers
A precipitate with HCl indicates either lead(II) or silver ions. Ammonia can then be added to produce different coloured solutions according to the cations present.
I hope this helps!
As established in the question, the identity of an ion can be determined by testing whether it forms precipitates and if it does, by noting the colour of the precipitate.So why are the other answers wrong
If I understand this correctly, ammonia needs to be added for confirmation. The other elements don't allow for this in this particular case, therefore making the other options incorrect.
I think they mean A, but it is a badly written question.
Consider option B. If I had copper(II) carbonate, I could add HCl and see it dissolve to form a blue solution, and I would get a pale blue precipitate when enough sodium hydroxide was added. If I had a white solid that was barium carbonate or magnesium oxide, both would dissolve in HCl but magnesium hydroxide would form much more readily as sodium hydroxide was added than would have barium hydroxide.
I think it is based on a single method of cation analysis that is in that book, but the syllabus does not mandate a particular method and there are multiple approaches that are practical.
Consider option B. If I had copper(II) carbonate, I could add HCl and see it dissolve to form a blue solution, and I would get a pale blue precipitate when enough sodium hydroxide was added. If I had a white solid that was barium carbonate or magnesium oxide, both would dissolve in HCl but magnesium hydroxide would form much more readily as sodium hydroxide was added than would have barium hydroxide.
I think it is based on a single method of cation analysis that is in that book, but the syllabus does not mandate a particular method and there are multiple approaches that are practical.




