The proper answer to this question goes beyond school level, but the short version is this:
Equilibrium constants are not actually based on concentrations, they are based on the activity of the species. In most common circumstances, the concentration of species dissolved in a solvent or in gas state is a very good approximation for the activity, which is why the simplification to concentrations is used. Solids and pure liquids (solvents) have activities of 1 and so they are not so much disregarded as constant as they are irrelevant as their value is 1.
Note that the "disregard pure liquids" rule runs into major problems when pure liquids are mixed. For example, if I mix ethanol and acetic acid as pure substances and then add a trace of (say) HCl as catalyst, the equilibrium that is established with have ethyl acetate and water as the products, and all four species will occur in the equation for
K. Water will not be the solvent, its concentration will change substantially if the system is disturbed, and you will have
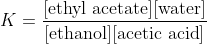
Species where the state is "(sol)" (meaning dissolved in a solvent other than water) will also be included in expressions for
K, while the solvent (with a "(l)" state) will be excluded. For example borane (BH
3) is usually used as a complex with a solvent like THF:
BH
3 (sol) + THF (l) <---> H
3B(THF) (sol)
would have
K = [H
3B(THF)] / [BH
3]
From an HSC perspective,
@Eagle Mum's explanation is the closest to the actual reason... the the negligible changes in concentration approximate the actual fixed activities.

