That's a good question, though not one that really fits inside the HSC syllabus.
Ring strain is a destabilising factor for ring systems except when they have six atoms in the ring -
Wikipedia has an article about it - and the problem with bond angles away from the ideal tetrahedral angle is intuitively obvious. The contribution from eclipsed rather than staggered conformations is also not difficult to appreciate in a qualitative sense, though the syllabus does not really go into those areas either.
The effect can be seen in its reactivity, for example. Cyclopropane reacts with HBr to yield 1-bromopropane, an addition reaction that is akin to the behaviour of propene but not propane.
Quantifying the effect of ring strain is well beyond the syllabus, though quantified effects can be seen in the elevation of the enthalpies of combustion. Estimating the enthalpy of combustion from bond energies for cyclopropane (in gas phase) gives:
Cyclopropane: C
3H
6 (g) + 4.5 O
2 (g) ---> 3 CO
2 (g) + 3 H
2O (g)
} + 6 \times \text{BE(C-H)} + 4.5 \times \text{BE(O=O)} - \left[6 \times \text{BE(C=O)} + 6 \times \text{BE(H-O)}\right] \\ &= 3 \times 348 + 6 \times 413 + 4.5 \times 495 - \left[6 \times 799 + 6 \times 463\right] \\ &= 5749.5 - 7572 \\ &= -1822.5\ \text{kJ mol}^{-1} \end{align*})
Adjusting back to standard states by using:
H
2O (g) ---> H
2O (l)
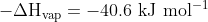
gives an estimate of the enthalpy of combustion as

.
The actual value is
} = -2091\ \text{kJ mol}^{-1})
.
This 150 kJ mol
-1 difference reflects that the three strained carbon-carbon bonds in the cyclopropane ring are weaker than a typical carbon-carbon bond. In fact, assuming that the strain only affects the carbon-carbon bond strength (which isn't true, but the effect on the carbon-hydrogen bond strengths will be significantly smaller in magnitude), we can calculate:
} + 6 \times \text{BE(C-H)} + 4.5 \times \text{BE(O=O)} - \left[6 \times \text{BE(C=O)} + 6 \times \text{BE(H-O)}\right] \\ -2091 &= 3 \times \text{BE(C-C in cyclopropane)} + 6 \times 413 + 4.5 \times 495 - \left[6 \times 799 + 6 \times 463\right] \\ -2091 &= 3 \times \text{BE(C-C in cyclopropane)} + 4705.5 - 7572 \\ \text{BE(C-C in cyclopropane)} &= \frac{-2091 + 7572 - 4705.5}{3} \\ &= +258.5\ \text{kJ mol}^{-1} \end{align*})
In other words, compared to the typical / average BE(C-C) = +348 kJ mol
-1, the carbon-carbon bonds in cyclopropane are only about 75% as strong.
There is the basis for a good, challenging, question that builds on content in modules 1, 4, and 7 (at least).
.
.

